Quantum Dots; Discovery and Properties
Quantum dots are nanoparticles that glow a particular color after being illuminated by light. They were first observed in 1980.
Discovery of Quantum Dots
In 1980 a Russian physicist named Alexi Ekimov discovered quantum dots in glass crystals. Ekimov worked for the Vavilov State Optical Institute. By late 1982 Louis E. Brus, a chemist at Bell Laboratories, found quantum dots in colloidal solutions. Brus found that quantum dots emitted or absorbed a wavelength of light. This wavelength changed over time as the nanocrystal grew.
Quantum Properties
When quantum dots are illuminated by light, the color they glow is determined by the size of the nanoparticle. When you illuminate quantum dots using UV light, some electrons get sufficient energy to break free from their atoms. This allows the electrons to move around the nanoparticle, which creates a conductance band. In this band electrons are free to move through a material and therefore to conduct electricity. When these electrons return to the valence band (the outer orbit around the atom), they emit light (see the following figure). The light’s color is determined by the energy difference between the conductance band and the valence band.
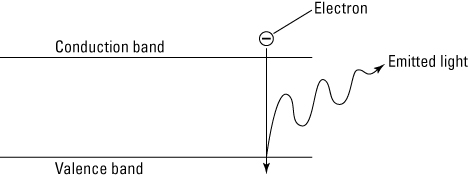
Electrons in a quantum dot generating light.
Depending on the size of a nanoparticle the energy difference between the valence band and conductance band will change. Smaller nanoparticles produce a higher energy difference, which results in a deeper blue color. Larger nanoparticles produce an energy difference between the valence band and the conductance band which is lower. This lower energy difference produces a glow that’s more red. Being able to control the size of quantum dots, allows control of the resulting colors.
A variety of semiconductor materials are used as quantum dots, such as cadmium selenide, cadmium sulfide, or indium arsenide. In fact, nanoparticles of any semiconductor substance have the properties of quantum dots because they contain the gap between the valence band and the conductance band. This characteristic is what causes quantum dots to fluoresce.
Uses of Quantum Dots
Quantum dots may be used to improve the efficiency of solar cells. In solar cells without quantum dots, a photon of light generates one electron. Researchers have found that both silicon quantum dots and lead sulfide quantum dots generate two electrons for every photon of light. That means that using quantum dots in solar cells could dramatically increase their efficiency for producing electric power.
People are also working on using quantum dots in displays used in products such as your cell phone or large screen television. Displays using quantum dots have the benefit of consuming less power than other displays. By placing different size quantum dots in each pixel of a display screen, the full spectrum of colors, made up of red, green, and blue colors, would be available.
Methods of Preparation
There are two ways to prepare quantum dots. You can form nanosized semiconductor particles using colloidal chemistry which involved an injection of semiconductor precursors into certain organic solvents. This method is usually used in biological systems.
The epitaxial growth method is used in products such as lasers or infrared photodetectors, both applications in the area of optoelectronics. Creating nanostructures using the epitaxial method involves crystalline nanostructures grown on a substrate. This creates a relationship between the nanostructures and the substrate. This method provides very precise control of the morphology, structure, and chemistry of nanostructures allowing them to have unique properties that are useful in several fields, such as electronics and energy.
Health and Safety
certain conditions are met, some quantum dots can be a risk to the environment and to people’s health. That toxicity could arise from several factors, including a particle’s physiochemical characteristics. Those characteristics could include size, shape, surface charge, and chemical composition, as well as factors determining their stability. Because of health concern about cadmium, quantum dots that contain cadmium are restricted in various countries and researchers are making quantum dots of various other materials to optimize safety as discussed on our Applications of Quantum Dots webpage.