On-demand vaccines possible with engineered nanoparticles
Vaccines combat diseases and protect populations from outbreaks, but the life-saving technology leaves room for improvement. Vaccines usually are made en masse in centralized locations far removed from where they will be used. They are expensive to ship and keep refrigerated and they tend to have short shelf lives.
University of Washington engineers hope a new type of vaccine they have shown to work in mice will one day make it cheaper and easy to manufacture on-demand vaccines for humans. Immunizations could be administered within minutes where and when a disease is breaking out.
“We’re really excited about this technology because it makes it possible to produce a vaccine on the spot. For instance, a field doctor could see the beginnings of an epidemic, make vaccine doses right away, and blanket vaccinate the entire population in the affected area to prevent the spread of an epidemic,” said François Baneyx, a UW professor of chemical engineering and lead author of a recent paper published online in the journal Nanomedicine.
The research was funded by a Grand Challenges Explorations grant from the Bill & Melinda Gates Foundation and the National Institutes of Health.
In typical vaccines, weakened pathogens or proteins found on the surface of microbes and viruses are injected into the body along with compounds called adjuvants to prepare a person’s immune system to fight a particular disease. But standard formulations don’t always work, and the field is seeking ways to manufacture vaccines quicker, cheaper and tailored to specific infectious agents, Baneyx said.
The UW team injected mice with nanoparticles synthesized using an engineered protein that both mimics the effect of an infection and binds to calcium phosphate, the inorganic compound found in teeth and bones. After eight months, mice that contracted the disease made threefold the number of protective “killer” T-cells – a sign of a long-lasting immune response – compared with mice that had received the protein but no calcium phosphate nanoparticles.
The nanoparticles appear to work by ferrying the protein to the lymph nodes where they have a higher chance of meeting dendritic cells, a type of immune cell that is scarce in the skin and muscles, but plays a key role in activating strong immune responses.
In a real-life scenario, genetically engineered proteins based on those displayed at the surface of pathogens would be freeze-dried or dehydrated and mixed with water, calcium and phosphate to make the nanoparticles. This should work with many different diseases and be especially useful for viral infections that are hard to vaccinate against, Baneyx said.
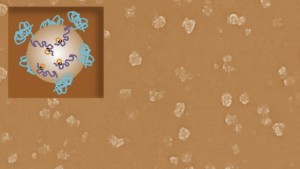 This image shows a collection of vaccinating nanoparticles, which at their largest are about 1,000 times smaller than a human hair. The inset graphic is a representation of how the engineered proteins decorate a nanoparticle’s surface.
This image shows a collection of vaccinating nanoparticles, which at their largest are about 1,000 times smaller than a human hair. The inset graphic is a representation of how the engineered proteins decorate a nanoparticle’s surface.
He cautioned, however, that it has only been proven in mice, and the development of vaccines using this method hasn’t begun for humans.
The approach could be useful in the future for vaccinating people in developing countries, especially when lead time and resources are scarce, Baneyx said. It would cut costs by not having to rely on refrigeration, and vaccines could be produced with rudimentary equipment in more precise, targeted numbers. The vaccines could be manufactured and delivered using a disposable patch, like a bandage, which could one day lessen the use of trained personnel and hypodermic needles.
Co-authors of the paper are Weibin Zhou, Albanus Moguche and David Chiu of the UW, and Kaja Murali-Krishna of Emory University.
University of Washington, January 12, 2014
--------------------------
---------------------------
