Related Articles on Targeted Chemotherapy using:
Tumor-Seeking Nanoparticles
Until recently, cancer researchers have taken a shotgun approach to killing cancer – administering drug that goes everywhere in the body with the hope that enough reaches malignant cells to kill them. The results of this approach are often serious side effects and suboptimal therapeutic response. But in order to develop more effective chemotherapeutics and imaging agents, scientists need to improve their aim—switching to a sniper’s rifle to deliver agents more accurately to tumors. Nanoparticles, with the ability to store large payloads within their cores and “targeting” molecules on their surfaces, would seem ideally suited to the task.
However, despite their name, nanoparticles are still bigger than many anticancer drugs. Their “large” size can make it difficult for them to evade organs such as the liver, spleen, and lungs, which are constantly clearing foreign materials from the body. In addition, they must be able to take advantage of subtle differences in cells to distinguish between normal and cancerous tissues. Indeed, it is only recently that researchers have begun to successfully engineer nanoparticles that can effectively evade the immune system and actively target tumors.
Active tumor targeting of nanoparticles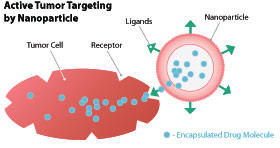 involves attaching molecules,
known collectively as ligands, to the outsides of nanoparticles. These
ligands are special in that they can recognize and bind to complementary
molecules, or receptors, found on the surface of tumor cells. When such
targeting molecules are added to a drug delivery nanoparticle, more of
the anticancer drug finds and enters the tumor cell, increasing the
efficacy of the treatment and reducing toxic effects on surrounding
normal tissue. In imaging applications, these materials can
guide more contrast agent to specific tissues, making even small tumors
in their formative stages more visible. As discussed below, several new
types of nanosystems are already revolutionizing the precision by which
cancer cells can be killed and tumors can be imaged.
involves attaching molecules,
known collectively as ligands, to the outsides of nanoparticles. These
ligands are special in that they can recognize and bind to complementary
molecules, or receptors, found on the surface of tumor cells. When such
targeting molecules are added to a drug delivery nanoparticle, more of
the anticancer drug finds and enters the tumor cell, increasing the
efficacy of the treatment and reducing toxic effects on surrounding
normal tissue. In imaging applications, these materials can
guide more contrast agent to specific tissues, making even small tumors
in their formative stages more visible. As discussed below, several new
types of nanosystems are already revolutionizing the precision by which
cancer cells can be killed and tumors can be imaged.
Nanoparticle-based Drug Delivery to Localized Prostate Cancer
Recently, a team of researchers led by Omid Farokhzad, M.D., of the Brigham and Women’s Hospital – Harvard Medical School, and Robert Langer, Ph.D., of the Massachusetts Institute of Technology, set out to determine if nanoparticle-based chemotherapy could offer some potential advantages over one conventional treatment for localized prostate cancer. Langer is co-principal investigator of the MIT-Harvard Center of Cancer Nanotechnology Excellence (CCNE) and Farokhzad is a project leader with the MIT-Harvard CCNE. Although recently published, the work is actually the culmination of many years of ongoing research. As Langer pointed out, “We have been developing nanoparticles for drug encapsulation and drug delivery for more than two decades. We have now built on that early work by developing a second generation of nanoparticles that can not only evade the immune system, but also target prostate cancer cells in a very specific way.”
Localized prostate cancer is often treated using brachytherapy, a treatment in which radioactive “seeds” about the size of rice grains are placed in the prostate to kill nearby cancer cells. Although many patients respond well to this treatment, approximately 10 percent fail brachytherapy. Moreover, some patients experience complications such as intestinal injury, incontinence, or impotence from the radiation exposure. The Farokhzad-Langer team was interested in determining how their new nanoparticles would perform in eliminating tumors as a potential alternative to the brachytherapy approach. “We wanted to see if nanoparticles could be given in one dose to produce significant tumor reduction without the complications that often accompany nonspecific radiation therapy,” explained Farokhzad.
For this application, the team designed customized nanoparticles that incorporated several safe or FDA-approved materials , each one performing a specific function in the final nanoparticle. They formed the nanoparticle’s backbone using a controlled-release copolymer that acted as a sponge to soak up and surround the chemotherapy drug docetaxel. The researchers gave the nanoparticle tumor-targeting capabilities by “decorating” the outside with short pieces of RNA that were designed to bind to specific proteins on the surface of prostate cancer cells. Hydrophilic, or water-liking, molecules known as poly(ethylene glycol), or PEG, were also incorporated into the nanoparticle’s outer shell to prevent the particles from binding to proteins in blood and attracting the attention of the immune system.
To test the effectiveness of these new drug-delivery devices, the investigators conducted experiments on mice bearing human prostate tumors. After waiting until the tumors had become large (to simulate the stage at which most prostate cancers are detected in humans), the researchers injected the nanoparticles directly into the tumors of the cancer-laden animals. The shrinkage in tumors after approximately three months was dramatic. Moreover, all of the treated mice survived the study. In contrast, only 57 percent of the animals treated with untargeted nanoparticles survived for the duration of the study, and only 14 percent of the animals treated with docetaxel alone survived.
These results indicated that the encapsulated drug did not move throughout the body, but rather stayed anchored to the tumor and delivered docetaxel to only cancer cells. Weight loss and white cell counts also confirmed lower toxicity of the treatment in mice that received the targeting nanoparticles versus those treated with untargeted ones. "A single injection of our nanoparticles completely eradicated the tumors in five of the seven treated animals, and the remaining animals also had significant tumor reduction, compared to the controls," said Farokhzad.
Targeting Disseminated Prostate Cancer
The next big challenge being pursued by these same researchers is the development of nanoparticles that can be given intravenously for targeting disseminated prostate cancer. This research will require nanoparticles that can get out of the bloodstream and into areas of the prostate tumor without being taken up by other organs. In the past, when nanoparticles have been given intravenously to target prostate disease, only about 1 to 2 percent of the initial dose ever reached the prostate gland.
How does one design nanoparticles that can evade everything in the body except cancer cells? No one knows for sure, so the Farokhzad group is pursuing two different approaches. The first involves randomly producing nanoparticles that have different combinations of size, charge, surface properties, and density of the targeting molecules. The investigators will then use high-throughput assays to screen for the nanoparticles that get taken up by one type of organ but not by another.
In the second approach, they are attempting to predict the preferred properties using computational methods, a process known as rational design. In the rational design process, the investigators will use parameters such as fluid sheer stress, drag force, and strength and density of tumor-binding molecules to try to determine what the optimum nanoparticles for a given application might look like.
Finding Tumors by Discovering Where They’re Not
In the meantime, researchers at the MIT-Harvard CCNE have already turned an obstacle into an opportunity by adopting an inverse targeting approach for imaging pancreatic cancer. They have designed nanoparticles that bind only to normal, as opposed to cancerous, pancreatic cells.
Pancreatic ductal adenocarcinoma (PDAC) is the fourth leading cause of cancer death in the United States. The mean survival average of only six months is due in part to limitations in diagnostics, which allows the disease to elude detection during its formative stages. What are needed are new contrast agents that can enhance the visualization of very small pancreatic tumors. Although many good nanoparticle-based contrast agents have been developed, most of them work by targeting molecules expressed only on the surface of tumor cells. Unfortunately, normal (noncancerous) pancreas tissue expresses many of these same molecules, making it difficult to distinguish tumors from healthy tissue.
Recently, a research team led by Lee Josephson, Ph.D., of Harvard Medical School and a member of the MIT-Harvard CCNE, and Ralph Weissleder, M.D.,co-principal investigator of the MIT-Harvard CCNE, figured out a way around this seeming difficulty. It was known from earlier work2 that there is a receptor protein, which binds the peptide bombesin, on the surface of normal pancreas cells that is absent on PDAC. It is this bombesin-binding protein on normal pancreas cells that they decided to target.
The Josephson-Weissleder team conducted experiments on mice with pancreatic tumors. By injecting nanoparticles with the bombesin peptide (ligand) attached to the surface into the mice tail veins, the relative signal coming from normal pancreas tissue was lowered. The tumors without the bound nanoparticles, on the other hand, showed up as relatively bright objects in subsequent magnetic resonance images.
History Repeated
The German scientist Paul Ehrlich, who coined the term chemotherapy at the beginning of the twentieth century, spent the third phase of his research career working on an idea from his thesis written when he was a young man. In that early work, he asserted that a drug’s chemical composition should be studied in relation to the affinity for the cells against which it is directed. Ehrlich spent his later years searching for compounds that could go straight to the organisms at which they were aimed. He called these elusive compounds “magic bullets.” It was not until he tested the 607th in a series that he found an arsenic-based bullet that proved effective in the treatment of human syphilis.
Although the past 30 years of innovation in nanotechnology has removed much of the “magic” to yield 21st century “smart bombs” capable of carrying a whole host of new anticancer drugs directly to tumors, we are still searching for the ideal delivery nanosystem. But unlike Erhlich, today’s researchers are armed with combinatorial, computational, and high-throughput screening methods to speed up the discovery process. More importantly, new developments in nanotechnology are continuing to fuel active and fruitful collaborations involving physical scientists, engineers, and cancer researchers, all of whom are passionate about adopting and designing nanosystems that can aid in the detection, treatment, and monitoring of cancer.
Source: The National Cancer Institute (www.cancer.gov); September 2006
Click here for info on targeted chemotherapy using spherical gold nanoparticles
-
Click here for info on targeted chemotherapy using nanoparticles called liposomes
_______________________
Advertisments
_______________________
--------------------------
---------------------------

