Silicon-coated Nanonets Could Build a Better Lithium-ion Battery
Unique nanotech structure demonstrates higher speed, capacity and longevity
A tiny scaffold-like titanium structure of Nanonets coated with silicon particles could pave the way for faster, lighter and longer-lasting Lithium-ion batteries, according to a team of Boston College chemists who developed the new anode material using nanotechnology.
The web-like Nanonets developed in the lab of Boston College Assistant Professor of Chemistry Dunwei Wang offer a unique structural strength, more surface area and greater conductivity, which produced a charge/re-charge rate five to 10 times greater than typical Lithium-ion anode material, a common component in batteries for a range of consumer electronics, according to findings published in the current online edition of the American Chemical Society journal Nano Letters.
In addition, the Nanonets proved exceptionally durable, showing a negligible drop-off in capacity during charge and re-charge cycles. The researchers observed an average of 0.1% capacity fade per cycle between the 20th and the 100th cycles.
“As researchers pursue the next generation of re-chargeable Lithium-ion battery technology, a premium has been placed on increased power and a greater battery life span,” said Wang. “In that context, the Nanonet device makes a giant leap toward those two goals and gives us a superior anode material.”
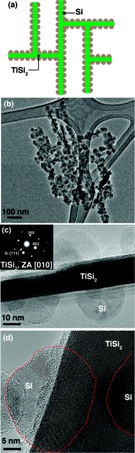 Frame (a) shows a schematic
of the Nanonet,
a lattice structure
of Titanium
disilicide (TiSi2), coated
with silicon (Si) particles
to form the active
component for Lithium-ion
storage.
Frame (a) shows a schematic
of the Nanonet,
a lattice structure
of Titanium
disilicide (TiSi2), coated
with silicon (Si) particles
to form the active
component for Lithium-ion
storage.(b) A microscopic view of the silicon coating on the Nanonets.
(c) Shows the crystallinity of the Nanonet core and the Si coating.
(d) The crystallinity of TiSi2 and Si (highlighted by the dotted red line) is shown in this lattice-resolved image from transmission electron microscopy. (Source: Nano Letters)
Lithium-ion batteries are commonly used in consumer electronics devices. This type of rechargeable battery allows Lithium ions to move from the anode electrode to the cathode when in use. When charged, the ions move from cathode back to the anode.
The structure and conductivity of the Nanonets improved the ability to insert and extract Lithium ions from the particulate Silicon coating, the team reported. Running at a charge/discharge rate of 8,400 milliamps per gram (mA/g) – which is approximately five to 10 times greater than similar devices – the specific capacity of the material was greater than 1,000 milliamps-hour per gram (mA-h/g). Typically, laptop Lithium-ion batteries are rated anywhere between 4,000 and 12,000 mA/h, meaning it would only take between four and 12 grams of the Nanonet anode material to achieve similar capacity.
Wang said the capability to preserve the crystalline Titanium Silicon core during the charge/discharge process was the key to achieving the high performance of the Nanonet anode material. Additional research in his lab will examine the performance of the Nanonet as a cathode material.
View the Nano Letters paper at http://pubs.acs.org/doi/abs/10.1021/nl903345f
Source: Boston University, News Release, February 15, 2010
The originator of a press release is responsible for it's content, not the UnderstandingNano Web site or Hawk's Perch Technical Writing, LLC
--------------------------
---------------------------
